
Authors
Sara C. Herstad, DO, Assistant Medical Director, CirrusMD, Denver, CO
Ted E. Palen, PhD, MD, Senior Investigator, CirrusMD, Denver, CO
Study Overview
Purpose
According to the Centers for Disease Control and Prevention (CDC), more than 60 percent of adults with asthma have poor asthma control, with the lack of adequate access to physicians for asthma care as one of the contributing factors. CirrusMD conducted a study to determine if patients with access to virtual care physicians could improve their asthma control.
Methods
CirrusMD performed a retrospective, observational study within a population of asthma patients who had access to primary care physicians through a secure, text messaging platform to manage asthma care. Baseline and follow-up Asthma Control Test (ACT™) questionnaire scores were used to measure asthma control. The Wilcoxon Signed-Rank statistical analysis was used to determine the before and after differences in ACT scores for each patient. The paired T-test was used to determine the Effect size and P-values.
Results
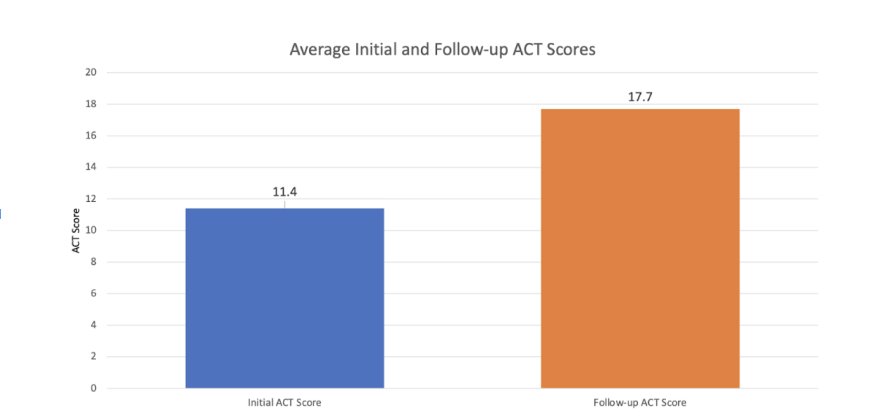
The patients displayed significant improvement to their asthma control and management. The average initial ACT score was 11.4 (range 6-19; 95% CI: 9.8, 13.0). The average final ACT score was 17.7 (range 6-24; 95% CI: 15.8, 19.5). The average difference between the initial and final ACT scores was 6.3 (95% CI: 4.2, 8.3). The ACT score difference was highly significant with a large Effect size of 1.22 and a P-value of < 0.0001.
Conclusions:
This study demonstrated that asthma patients managed by primary care physicians using a virtual care modality can significantly increase the control of their asthma as measured by their ACT scores.

Introduction: Asthma Chat ACT Study
Asthma is a chronic inflammatory disease of the airways that affects from 20 to 26 million adults in the United States.(1) Only 50% of adults with asthma have their asthma under control.(2) To keep their asthma under control, patients need to self-monitor their breathing and understand triggers that aggravate symptoms. In addition, they need regular monitoring by healthcare professionals to ensure that they are using appropriate medications to decrease exacerbations.(3-6) Adding an asthma controller medication can improve breathing, decrease exacerbations, and improve quality of life for asthma patients.(3)
The Asthma Control Test™ (ACT)(7) is an important tool for monitoring asthma control.(8) An ACT score of 5 indicates poor asthma control while a score of 20-25 indicates good asthma control.(9) An increase in the ACT score of 3 or more indicates a significant clinical improvement in asthma control.(10)
Patients often do not have adequate access to physicians, education, and appropriate medications to help them control their asthma; a situation only worsened by the COVID-19 pandemic. CirrusMD provides patients with virtual primary care delivered by board-certified physicians using its secure chat (text messaging) platform. It is available as a healthcare benefit to employees of participating self-insured companies, and members of participating commercial and government-sponsored health plans, including the U.S. Veterans Health Administration and Medicare.
To assess asthma control in a virtual care setting, we used ACT scoring to determine if asthma management occurring between patients and physicians via secure text chat could improve patients’ ACT scores and thereby increase asthma control for these patients.

Methods
CirrusMD physicians use virtual care modalities to care for patients with acute care, primary care, and mental health concerns. Patients securely log into the service and begin engaging with a physician in under 60 seconds using a secure, real-time texting (chat) function to discuss their medical concerns.
The study performed was a retrospective, observational study within the patient population using the CirrusMD chat-first, integrated care modality during the study period from August 24, 2021 through March 2, 2022.
Throughout this period, the CirrusMD primary care team of family medicine physicians interacted with patients on the platform. When the reason for a patient’s encounter was determined to involve asthma, the patient was provided a link to the ACT questionnaire(11) and asked to complete it to determine their initial score. Once the questionnaire was complete, patients were asked to provide the CirrusMD physician their score or upload a screenshot of their score.
Based on their answers to the ACT questionnaire, their asthma medical history, and their asthma medications, the treating physician created an asthma care plan for the patient. This included asthma care education and medication adjustments or additions. Once a care plan was established, one or more follow-up chat sessions were scheduled, at which time patients were asked to complete another ACT questionnaire. The initial and final ACT scores were collected and tabulated, in addition to the dates of the chat sessions. The patient’s age and gender, which were also collected.
A clinically significant increase in the ACT score is three points.(10) A sample size of 21 patients was needed to reach an Effect size of 0.8 for an increase in three points of the ACT score. We used Wilcoxon Signed-Rank statistical analysis to determine the before and after differences in ACT scores for each patient. The differences between the initial and final ACT scores were normally distributed. Therefore, the paired T-test was used to determine the Effect size and P-values.
Results
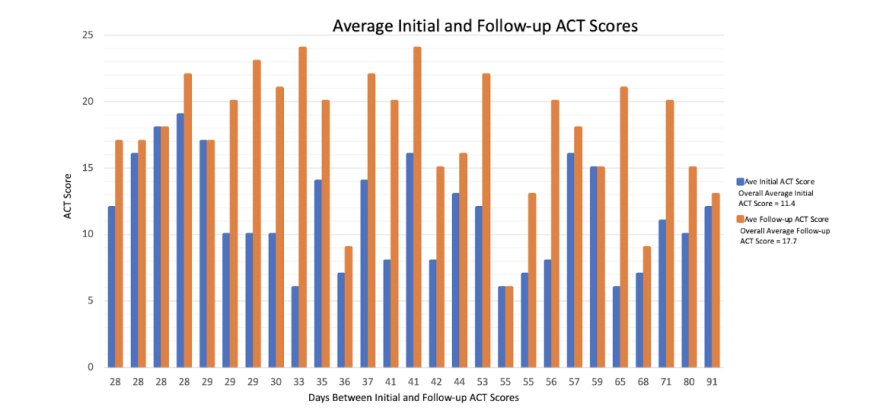
During the study period, 27 patients completed both the initial ACT score and at least one follow-up ACT score 28 or more days after the initial assessment (after censoring 3 patients who had upper respiratory infections). The average age of the patients was 33.3 years, with females comprising 74.1% of the participants. The average time between the initial and final ACT score determination was 42.1 days (range 28-91 days).
The average initial ACT score was 11.4 (range 6-19; 95% CI: 9.8, 13.0). The average final ACT score was 17.7 (range 6-24; 95% CI: 15.8, 19.5). The average difference between the initial and final ACT scores was 6.3 (95% CI: 4.2, 8.3). The ACT score difference was highly significant with a large Effect size of 1.22 and a P-value of < 0.0001.
Twenty-five patients had an initial ACT score ≤17. These patients had an average ACT score of 10.8 (95% CI: 9.4, 12.3) and a final ACT score of 17.5 (95% CI: 15.5, 19.5). The average ACT difference was 6.6 (95% CI: 4.5, 8.8. The Effect size was 1.3 and P-value < 0.0001.
Twenty-three of the 27 (85.2%) patients had a change in their asthma medication, based on asthma treatment standards. These changes included: 17 patients had an asthma controller medication added, switched, or dose increased, one patient had a controller medication added along with a nicotine replacement medication, two patients had a leukotriene medication added, three patients had the addition of both a controller medication and a leukotriene medication, and four patients had no medications additions or changes and were encouraged to use their controlled medication regularly.

Discussion
Patients with asthma often have difficulty controlling their symptoms. The use of the CirrusMD chat function improves asthma patients’ ability to obtain care. An improvement in the asthma ACT score of 3 or more is considered clinically important.(10) An ACT score of 20 or more indicates asthma is under control. The patients enrolled in the CirrusMD study had an average ACT score improvement of more than 6 points. Among the patients with initial ACT scores less than 18, the ACT score improvement was nearly 7 points.
The study had several limitations and strengths. One limitation was the lack of a traditional control group, however each patient served as their own control in the statistical analysis methodology that was used. It was determined that at least 21 patients needed to enroll for the study to realize an Effect size of at least 0.8 for an ACT score increase of three points. With 27 patients ultimately enrolled, an average ACT score increase of over six points was registered, which achieved a highly significant Effect size.
Another potential limitation is that the study took place during the COVID-19 pandemic so asthma patients may have been more isolated and subjected to fewer asthma triggers. However, the initial ACT scores indicated that these patients had uncontrolled asthma despite potentially being exposed to fewer triggers. Access to CirrusMD virtual primary care for these patients was subsidized as part of their employee benefits or health plan member benefits, so this group may have been more motivated. Therefore, our findings may not be generalizable to all asthma populations.
After patients demonstrated control of their asthma symptoms, they were provided with a 6-month supply of their controller medication and encouraged to follow up with their primary care physician. Patients were encouraged to use discount coupons to help make the cost more affordable when appropriate, however working with pharmacies to find a covered medication proved to be difficult for many patients.
Conclusion
As this study demonstrated, patients using a virtual primary care modality can significantly increase the control of their asthma as measured by their ACT scores during a one-to-three month time period. Patients reported feeling much better overall, which should positively impact continued adherence to the prescribed treatment regimen. Additional research is needed to determine if virtual care management of asthma patients translates into long-term asthma control.
Appendix
Asthma Control Test (ACT)
Used by permission from GlaxoSmithKline
Abbreviations: ACT: Asthma Control Test, CI: confidence interval, COVID-19: Corona Virus Disease 2019 caused by SARS-CoV-2
References
Centers for Disease Control and Prevention. 2016 Asthma Study data. https://www.cdc.gov/asthma/asthma_stats/uncontrolled-asthma-adults.htm
Centers for Disease Control and Prevention. 2019 National Health Interview Survey data. U.S. Department of Health & Human Services. www.cdc.gov/asthma/nhis/2019/data.htm
Centers for Disease Control and Prevention. (2018). AsthmaStats: Uncontrolled Asthma among Persons with Current Asthma. U.S. Department of Health & Human Services. Retrieved from: www.cdc.gov/asthma/asthma_stats/uncontrolled_asthma.htm (Accessed on March 12, 2022)
National Asthma Education and Prevention Program: Expert panel report III: Guidelines for the diagnosis and management of asthma. Bethesda, MD: National Heart, Lung, and Blood Institute, 2007. (NITH publication no. 08-4051). www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm (Accessed on March 12, 2022).
Expert Panel Working Group of the National Heart, Lung, and Blood Institute (NHLBI) administered and coordinated National Asthma Education and Prevention Program Coordinating Committee (NAEPPCC), Cloutier MM, Baptist AP, et al. 2020 Focused Updates to the Asthma Management Guidelines: A Report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group. J Allergy Clin Immunol 2020; 146:1217.
Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention. www.ginasthma.org (Accessed on March 12, 2022).
British Guideline on the Management of Asthma. www.brit-thoracic.org.uk/guidelines-and-quality-standards/asthma-guideline/ (Accessed on March 12, 2022).
Asthma Control Test™ (ACT). Open access to ACT is available at www.asthma.com/understanding-asthma/severe-asthma/asthma-control-test.
Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, Murray JJ, Pendergraft TB. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004 Jan;113(1):59-65.
Schatz M, Sorkness CA, Li JT, Marcus P, Murray JJ, Nathan RA, Kosinski M, Pendergraft TB, Jhingran P. Asthma Control Test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol. 2006 Mar;117(3):549-56. doi: 10.1016/j.jaci.2006.01.011. PMID: 16522452.
Schatz M, Kosinski M, Yarlas AS, Hanlon J, Watson ME, Jhingran P. The minimally important difference of the Asthma Control Test. J Allergy Clin Immunol 2009;124(4):719-723 e1, Epub 2009/09/22. www.asthma.com/understanding-asthma/severe-asthma/asthma-control-test/
Authorship Confirmation Statement
T.E.P. conceived of the study and study design. S.C.H. oversaw data collection. T.E.P. performed the statistical analyses. T.E.P. and S.C.H. discussed the results and wrote the final manuscript.
Acknowledgements
We wish to thank Donna Baldwin, DO, Chief Quality and Innovation Officer for CirrusMD, Elishia M. Oliva, MD, Medical Director, CirrusMD, and Justin Chang, MD, Vice President of Provider Network, CirrusMD, for supporting this project and for their suggestion to improve the manuscript.
Disclosure Statement
The authors have no conflict of interests.
Funding Information
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
There have not been any prior presentations of the material.







